
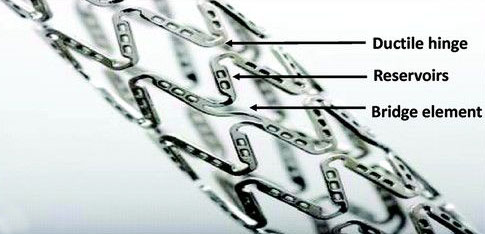
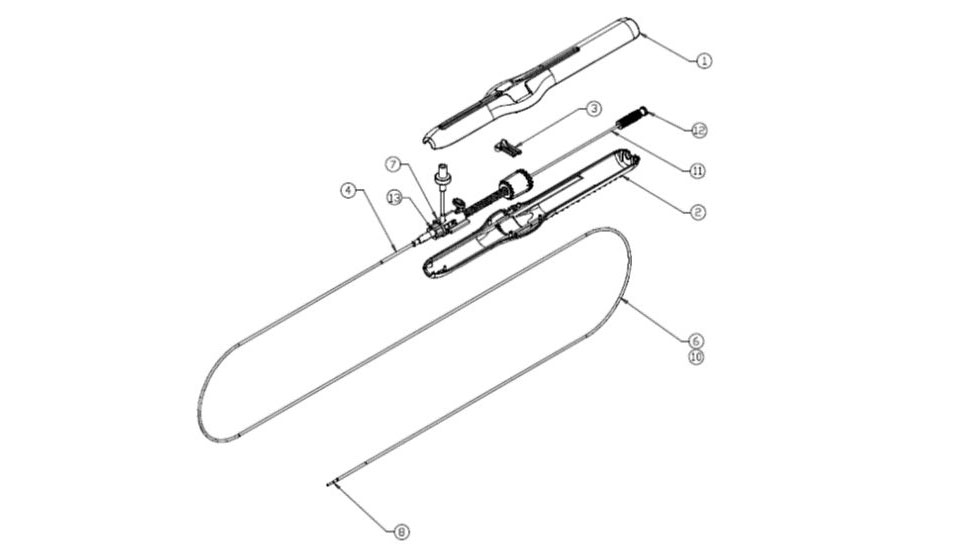
Design & Development of drug eluting coronary stent and stent delivery system to reduce the occurrence of stent thrombosis
Speed to Market, Design Complexity, Novel material/technology
Compliance to internal & external standards including ISOs: 14971, 10555, 25539, 10993, FDA guidelines for stents and delivery systems
Design Evaluation, Design Characterization, Design Verification & Validation
Component Qualification & supplier qualification
Investigational testing & comparative studies per ASTM standards: D638, E8, F3067, F2477, F3067, F2942
Statistical Process Capability and Data Analysis using Minitab
Deliverables: Design Dossier, DHF, dFMEA, pFMEA, Protocols & Reports
EZEN is a provider of digital transformation services and solutions to the healthcare industry, serving providers, life sciences (pharmaceuticals and medical devices), and technology firms (serving provider and life sciences markets).
EZEN makes an ideal partner for organisations looking at both transactional and transformational IT and Digital solutions because of its core capabilities, great human resources, commitment to quality, and the infrastructure to deliver a wide range of technology, consulting, and global staffing solutions and services, 24/7.