
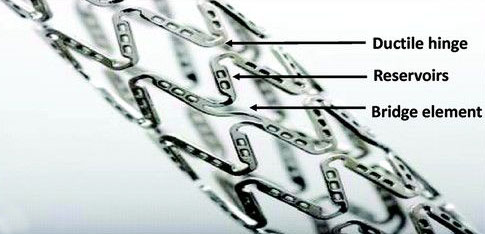
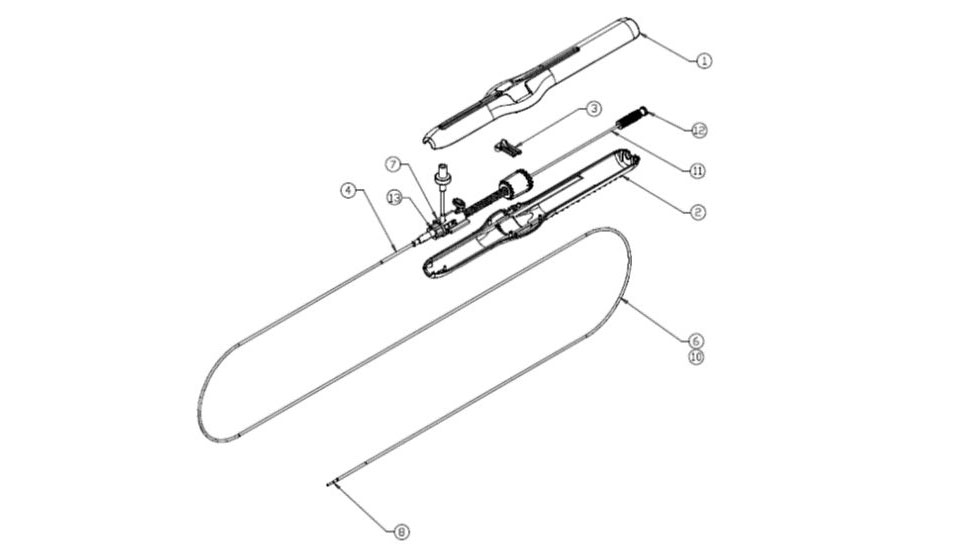
Design & Development of drug eluting coronary stent and stent delivery system to reduce the occurrence of stent thrombosis
Speed to Market, Design Complexity, Novel material/technology
Compliance to internal & external standards including ISOs: 14971, 10555, 25539, 10993, FDA guidelines for stents and delivery systems
Design Evaluation, Design Characterization, Design Verification & Validation
Component Qualification & supplier qualification
Investigational testing & comparative studies per ASTM standards: D638, E8, F3067, F2477, F3067, F2942
Statistical Process Capability and Data Analysis using Minitab
Deliverables: Design Dossier, DHF, dFMEA, pFMEA, Protocols & Reports